Membrane trafficking is important for communicating with other cells and acquire resources.
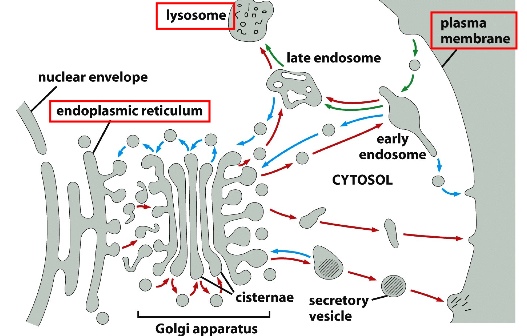
Basic Principles of the Biosynthetic-Secretory (going out) and Endocytic Pathways (coming in)
1) Protein Trafficking Routes (polarized trafficking that send proteins in one direction)
2) Sorting Stations: different materials from various places converge at one point and get sent out to different destinations
3) Retrieval Mechanisms and General Balance: endocytosis is balanced with exocytosis
Exocytosis: 2 kinds of secretory pathways:
- constitutive: cargos are continuously being packaged and sent out of the cell
- regulated: cargos re packaged but wait for specific signals before being sent out
^^ in both pathways, the basic mechanism is similar: cargo aggregate in Golgi —> transported to trans Golgi network —> cargo containers can bud off as loosely bound, immature secretory vesicles —> multiple containers like these can fuse together to concentrate the cargo —> extra membrane is recycled back to Golgi by clathrin coat —> mature secretory vesicles
See slides for electromicroscopic graphs of both pathways. What’s important is that in both pathways, cargo needs to be concentrated first. Then in constitutive pathway, the vesicle dock with the plasma membrane where both types of membrane fuse and release cargo; in the regulated pathway, the vesicles are then stored until a signal is detected.
Regulated secretion can provide plasma membrane(PM) when it’s been depleted: i.e. during cell division where extra PM is required; phagocytosis where PM becomes vesicle membrane; wound repair using extra PM.
Endocytosis:
- pinched off —> fuse with early endosome —> from there can 1) go back to plasma membrane (recycle); 2) be sent to late endosome(degradation) —> and destroyed in lysosome; 3) fuse with other intracellular membranes (transcytosis)
- e.g. phagocytosis: cells can internalize pathogens by pulling them inside and digest them
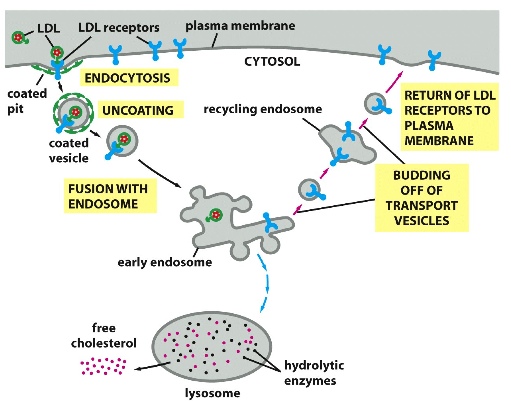
- e.g. collecting cholesterol: LDL (made up of cholesterol and proteins) that are flowing in the bloodstream bind to LDL receptors on cell surface —> PM forms vesicles that endocytose LDL along with its receptors —> vesicles fuse with early endosome (a sort ing station where the components can then be sent to different places) —> LDL receptors go back to PM to continue collecting LDL, but LDL are sent to lysosomes where they can be degraded
Local membrane change:
> exocytosis - involves fusion of membrane
***Grey = cytosol; red membrane = vesicle membrane; grey membrane = PM; white space outside = extracellular space; white space inside = vesicle lumen
Here, the vesicle lumen is never exposed to the cytosol. Once the fusion is complete, the cargo (lumen portion) becomes part of the extracellular space. Basically the aqueous content of the lumen can be seen as equivalent with the aqueous content of the extracellular space.
> endocytosis- involves invagination of membrane
***Grey = cytosol; red membrane = vesicle membrane; grey membrane = PM; white space outside = extracellular space; white space inside = vesicle lumen
Again, extracellular content can become lumen content when a vesicle forms.
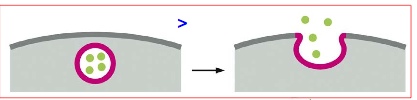
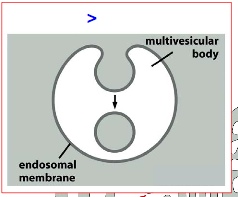
> degradation- involves budding of membrane
***Grey = cytosol; white space outside = extracellular space; grey membrane = endosomal membrane; white space inside = multi vesicular body lumen; grey space inside = equivalent to cytosol content(note the difference here)
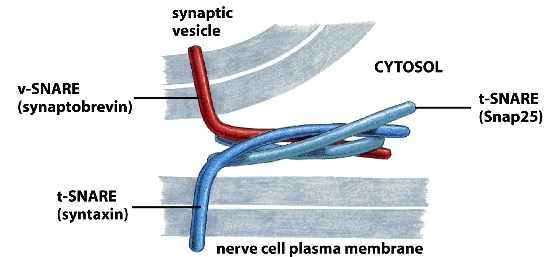
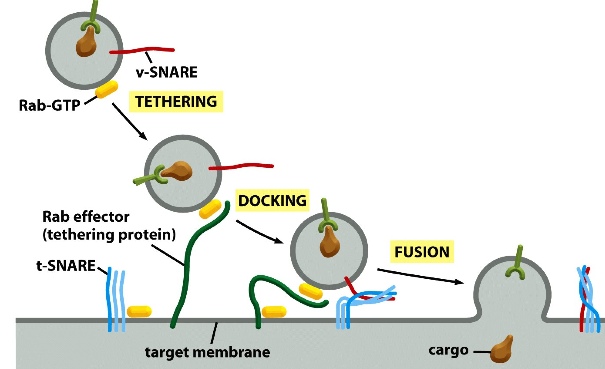
SNARE:
exocytosis-fusion
v-SNARE on vesicles
t-SNARE on target membrane
When v-SNARE binds to t-SNARE and intertwine together, it forces the vesicle to fuse with its target membrane
The important concept here is that the outer leaflets of both membranes fuse first, forming a stalk like structure which exposes the inner leaflets of both membrane —> inner leaflets of both membrane fuse, exposing the cytosol
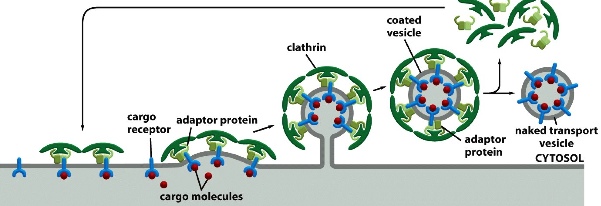
exocytosis-invagination
clathrin coat + dynamin
(COPI and COPII also drive invagination)
Cargo receptors select cargo from the cytosol —> adaptor proteins that are bound with clathrin coat then pick up receptors —> more and more clathrin-adaptor form a vesicle —> once the vesicle is formed, clathrin coat is shed
Clathrin is made up of 3 heavy polypeptide chains and 3 light chains — gives the curvature of the vesicle, helps it form a dome like structure
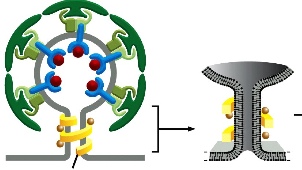
At the end of vesicle forming, a protein called dynamin and its associated protons force these two bilkers close together so that they fuse and thus closing up the vesicle.
budding
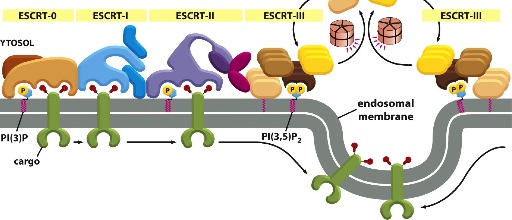
ESCRT
Not well understood, but it is involved in binding cargo , forming cargo vesicles and fusing vesicle membranes together.
***note that in this diagram there are in fact only 2 green cargo receptors: one from the left and another one from the right. The green cargo receptors is ubiquitylated, allowing them to be bound to different ESCRT complexes. From left to right we can see ESCRT-0 binding to cargo —> unbinds and passes cargo to ESCRT-1 —> ESCRT-1 unbinds and passes cargo to ESCRT-II —> ESCRT-II unbinds and passes it to ESCRT-III —> ESCRT-III from both left and right form an extensive network that traps cargo in the middle of the membrane, which then invaginate into vesicles.
Regulation of membrane transport machinery
- SNARE: draw specific membrane together
- signalling lipids (PIPs):
-
- derived from inositol phospholipids
- the inositol sugar head group has many -OH groups that can be modified — in this case, they are phosphorylated. A variety of PIPs can be made by adding -PO4 at different places (multiple combinations)
- these PIPs act as specific markers on the membrane surface. Each PIP species binds to specific proteins —> thus creating specificity for binding throughout the trafficking network (provides an address)

- structure of a simple PIP:

- Small GTPases
-
- ON when it’s bound to GTP (GEFs turn it on)
- OFF when it’s bound to GDP(GAPs turn it off)
- are specifically localized in the cell
- one major type of small GTPases is the Rab small GTPases:
-
- Rab5 (located in PM, clathrin-coated vesicles, early endosome)
- Rab 7 (located in late endosomes: direct cargo to lysosomes)
- Rab 11 (located in recycling endosomes)

> Rab5-GDP has unexposed amphipathic helix —> Rab5-GEF activates Rab5 and exposes the helix —> Rab5-GTP can recruit more Rab5-GEF for further activation
> Rab5-GTP can also work with PIPs to further specify the cargo destination
Rab-GTP on the cargo vesicles associate with Rab effector (tethering protein on target membrane) —> tethering proteins draw the vesicle close to the membrane —> SNAREs on both side bind together to force the two membrane to fuse together
We still don’t know how GEF or GAP tells Rab where to go.